You’ve had an injury to one of your limbs and your bone is fractured. As you face the challenges of a long road to recovery, and the ways in which it will impact your life, does the idea of taking a magic pill to speed up your bone healing sound too good to be true?
That’s what some Sunnybrook scientists are questioning, and now investigating in a clinical research study to enhance bone fracture repair.
The “magic pill” in this case is a daily medication already scientifically tested and well established in clinical use known as lithium carbonate.
With timed administration at very low doses, lithium carbonate has been shown to enhance bone repair, and now, orthopaedic clinical researchers are testing it in injured patients with fractures.
The Sunnybrook-led study – Lithium for Fracture Treatment (LiFT) – is a randomized controlled multi-centre clinical trial investigating if lithium can improve long bone fracture healing in healthy patients from 18 to 55 years of age, who have a shaft fracture of the femur, tibia/fibula, humerus or clavicle. Participants take the medication for just two weeks starting 14 days after the fracture or surgery.
“Fracture healing in adults typically takes several weeks to months to heal – and in up to 10 per cent of cases, it can fail to heal completely despite appropriate treatment,” says Dr. Diane Nam, an associate scientist with the Holland Bone and Joint Research Program and orthopaedic trauma surgeon at Sunnybrook.

Dr. Diane Nam, Principal Investigator on the LiFT trial.
“Sustaining a fracture significantly impacts an individual’s function, such as the ability to work, drive or physically parent young children. We’re talking not only about the long time to heal bones, but don’t forget the pain and negative impact on a patient’s quality of life and mental health. If we can show that this safe and inexpensive ($1/a day) therapy works, it can potentially have a huge impact to globally change how we manage fractures.”
Dr. Nam was involved in the scientific research from “lab bench to patient bedside”. She led the team as its principal investigator in conducting the pre-clinical work and in translating the research through to the current clinical trial.
The first few patients in the initial pilot clinical trial showed a reduction in pain with the use of lithium, with no impact on mental activity or mood. In order to keep the highest standards for clinical research, the current study is blinded, meaning that patients and the research team who will analyze the data do not know who is receiving the actual drug or a placebo pill in order to control for potential bias. 120 patients have been enrolled in the study to date.
The trial has sites at Sunnybrook, St. Michael’s Hospital, McMaster University Medical Centre, Ottawa Hospital and Royal Victoria Regional Health Centre, and is supported through Sunnybrook’s Centre for Clinical Trial Support (CCTS) and the Canadian Institutes for Health Research.
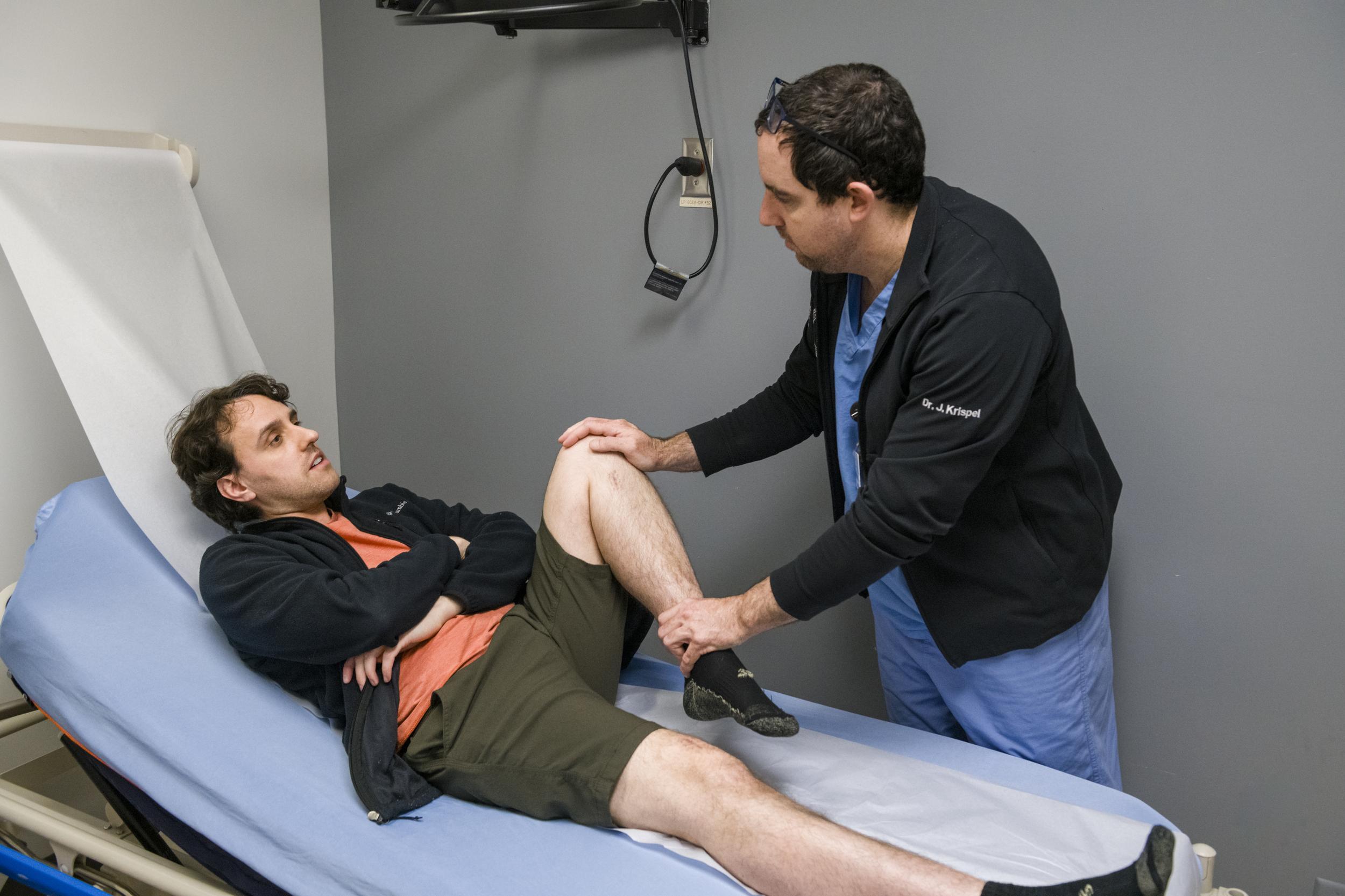
A research participant from the LiFT trial comes in for one of his check-in visits at the fracture clinic and to have x-rays taken.
The research team will analyze and compare X-ray result scores assessing for bone union where three of the four outer layers at the fracture site are bridged. They will also factor in how the research participants are functioning in their day-to-day life, including accounting for pain levels.
The research is also considering potential barriers to the acceptance of lithium therapy for the purpose of fracture healing, from both the perspective of patients and care providers. Of note, the dose of lithium being used in the study is so low (300 mg or one-quarter of the dose amount used for other applications) that it’s not detectable in blood work at 12 hours after taking it.
“This important clinical trial is one of many promising investigations that have resulted from collaborations between surgeons and scientists in the Holland Bone and Joint Program,” says Dr. Cari Whyne, senior investigator involved in the trial and the Susanne and William Holland Chair in Musculoskeletal Research at Sunnybrook Research Institute and University of Toronto. “This aligns with my research focus in clinically-translational bioengineering research aimed at maximizing function among those who develop musculoskeletal disease or disability.”
For more information about participating in the LiFT trial, contact lift@sunnybrook.ca