Informally dubbed “Critical Care Central,” Sunnybrook Health Sciences Centre cares for approximately 2,000 of the most seriously-injured and sickest patients each year; the highest number in Canada.
It’s this highly-specialized setting within an academic health sciences centre that lays the foundation for clinical research – the study of health and illness in people – that drives discovery, innovation and learning in the field of critical care, at home and around the globe.
According to the Canadian Clinical Research Network, for the second year in a row, Sunnybrook has enrolled the highest number of patients in Canada in critical care-related trials funded by the Canadian Institutes of Health Research (CIHR).
Two current Sunnybrook-led clinical trials – on the cusp of discovery – are expected to produce evidence that will change the way critical care is practiced, and impact outcomes for critically-ill patients:
The world’s largest clinical trial in critical care:
Using antibiotics to prevent hospital-acquired infections before they happen.

Dr. Brian Cuthbertston, senior scientist in the Evaluative Clinical Sciences, Tory Trauma Research Program at SRI, is the international principal investigator for a number of global critical care studies and collaborations, including the SuDDICU trial.
Critically-ill patients who receive mechanical ventilation (assisted breathing) in an intensive care unit (ICU) are particularly at risk for hospital-acquired infections – these are infections that can develop while patients are in hospital receiving care, and are a major cause of illness, sometimes death, and increases to the costs of care.
While the evidence supporting the preventative use of antibiotics is strong, many health care professionals around the world don’t use this approach, out of a concern of the effects of antibiotic resistance – when antibiotics are no longer effective.
Led by SRI in Canada and the U.K., and by The George Institute in Australia, the SuDDICU study – Selective Decontamination of the Digestive tract in Intensive Care Unit patients – is a large, randomized controlled trial and international research collaboration, that was first established in 2009.
The researchers wanted to test:
- whether using antibiotics to prevent infections increases the number of patients who get better and go home after being critically unwell, and
- whether using antibiotics in this way affects patterns of antibiotic resistance – when antibiotics are no longer effective – in the ICU.
“The preliminary results are not only promising, but also came as a bit of a surprise,” says Dr. Brian Cuthbertson, international principal investigator of the trial, critical care physician and senior scientist at Sunnybrook.
This research aims to give health care professionals and patients data on the benefits so they can make informed decisions about providing preventive antibiotics as part of care.”
Publication of the study results are expected in the near future – stay tuned to SRI Research News.
The clinical trial ranked #1 out of hundreds of thousands of Health Canada pandemic study grant applications:
Swapping intravenous (IV) sedative for inhaled sedative for patients on a ventilator in the ICU.
This trial is homegrown within Canada (and includes a single U.S. site), but its potential for global impact is not any less significant.
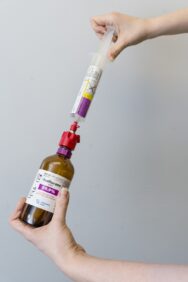
A dose of isoflurane is prepared, as part of the SAVE-ICU trial designed and led by Sunnybrook. The inhaled sedative – known for its use in surgeries – has been tested for use with patients in the intensive care unit, versus traditional sedatives given through an intravenous (IV) line.
The SAVE-ICU study – SedAting with Volatile Anesthetics Critically Ill COVID-19 Patients in the Intensive Care Unit – is a collaborative, multi-hospital, randomized clinical trial testing:
- whether inhaled volatile sedatives – a more widely available anesthetic commonly used in operating rooms – can replace sedative drugs that are typically delivered intravenously (by IV) for patients in the ICU with respiratory distress requiring ventilation; and
- whether patients recover faster with this form of sedation.
“In order to tolerate the uncomfortable procedure of being put on a breathing machine, patients require sedation or sleep-inducing medications,” explains Dr. Angela Jerath, lead principal investigator of the study, anesthesiologist, and a scientist in Evaluative Clinical Sciences at Sunnybrook. “At the beginning of the COVID-19 pandemic, these drugs were in short supply due to the high number of patients needing ventilators.”
The investigators will compare the impact that inhaled versus IV sedation has on outcomes important to patients with respiratory failure, their ICU clinical teams, and health resource use; this includes ICU and ventilator-free days, quality of life, delirium and hospital mortality.
Adds Dr. Jerath: “There has been some evidence to suggest that these (inhaled volatile) drugs may also have properties that reduce lung inflammation, which may speed up recovery and reduce the time patients spend on a ventilator.”
The investigators are also continuing to look for any elevation in risk between the two methods of sedation, but there has been no indication to stop in the last few years, with no adverse events showing.
“As the inhaled sedative doesn’t have to be filtered through the body like the IV sedation does, there are benefits for the lungs, liver and kidneys, for cancer patients, and with no particular concerns over IV sedation seen,” explains Eily Shaw, the research coordinator on the trial.
“When you turn the dose up/down with an IV drip, you need to wait for the body to process it first, and there’s also a considerable ‘wash out’ period after with patients coming off the effects from the sedative days or even weeks later. It can be a long time before they feel themselves again which can be confusing and sometimes scary.”
Although volatile (inhaled) anesthetics are not new (as a standard of care for surgeries), “their use still had to be studied in the context of the ICU because the type of person coming in for surgery is different than the patient who is ventilated due to respiratory distress in critical care,” says Eily.
At the study start in 2021, it was initially aimed at patients with a COVID diagnosis, but has since expanded to include any patient in the ICU with any kind of respiratory distress (lung failure) requiring sedation on a ventilator.

Dr. Angela Jerath, scientist and anesthesiologist in the Schulich Heart Program, is the lead principal investigator of the SAVE-ICU study.
The goal of this study is to determine if inhaled sedation should be a standard of care in the ICU as well,” says Dr. Jerath. “This can be done differently between hospitals (with different tiers of care), and would also ease the pressure on IV sedation stock, in particular during heightened times of need, such as a pandemic.”
With over 750 research participants over the four-year study period, it will take another year or two to assess the data. In the meantime, members of the research team like Eily are left humbled with their own anecdotal personal observations and sense of what it means:
I’m always buoyed by how thankful our participants and their families are to be involved in our research. For me, the human component is what makes the work we do particularly special. Our team certainly can’t take all the credit but it’s so massively rewarding to see patients when they get better and they look totally different at follow ups and are so grateful, we understand the importance of doing this.”